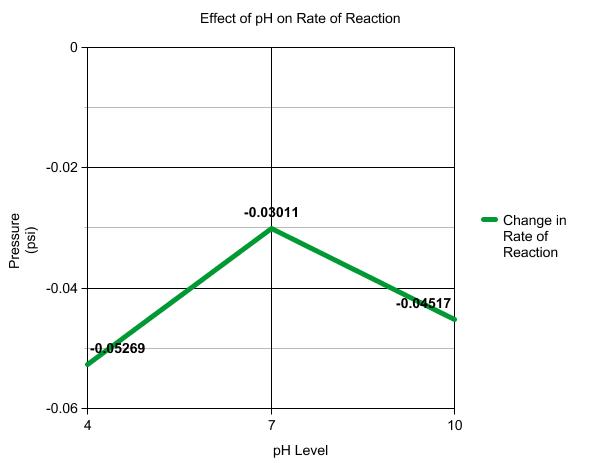
Photosynthesis. Or, How to make your own food.......
Essentially, photosynthesis is the process by which photoautotrophs synthesize food (sugar) from carbon dioxide (CO2) using the energy from light. Sounds mighty dang simple right? Well, it mostly is. It happens like this: In oxegenic photosynthesis (literally photosynthesis that releases oxygen) Light energy is absorbed by proteins that contain chloroplasts which contain chlorophylls (by the way, chlorophylls are green because that is the part of the light spectrum they do not use). The chlorophylls store most of the energy in the form of ATP.
The next step in photosynthesis is the oxidation of H20. Energy (ADP) is used to split the hydrogen and Oxygen molecules apart. The oxygen molecules are released (they can be used in respiration), and the hydrogens are kept. When CO2 is added to the process they each lose an Oxygen, which attach to some of the hydrogen molecules, forming H2O. The remaining Hydrogen, Oxygen, and Carbon then all get combined into glucose.
 |
| Chloroplast |
The next step in photosynthesis is the oxidation of H20. Energy (ADP) is used to split the hydrogen and Oxygen molecules apart. The oxygen molecules are released (they can be used in respiration), and the hydrogens are kept. When CO2 is added to the process they each lose an Oxygen, which attach to some of the hydrogen molecules, forming H2O. The remaining Hydrogen, Oxygen, and Carbon then all get combined into glucose.
---CO2 can also be converted into sugars suing a process called carbon fixation. The most common type of carbon fixation in biological life is the Calvin cycle. The Calvin cycle describes the way that light energy is used in the creation of chemical free energy, stored in glucose. The key enzyme that makes the cycle run is called RubBisCO, found in the chloroplast stroma. The Calvin cycle includes a number of regulatory functions that prevent it from being respired (look at the second half of the post) into CO2 (preventing energy (ATP) from being wasted without a net gain). For more information on the Calvin cycle, click here.-----
The glucose can then be burned through cellular respiration.
Cellular Respiration Or:how you use your food
Cellular respiration is the process by which nutrients are broken down into ADP. The first kind of respiration is called aerobic respiration. Aerobic respiration requires oxygen to break down the nutrients. It has three main stages. Glycosis, Krebs cycle, and electron transport.
Maximum ATP Yields: Prokaryotic cells can yield a maximum of 38 ATP molecules while eukaryotic cells can yield a maximum of 36. In eukaryotic cells, the NADH molecules produced in glycolysis pass through the mitochondrial membrane, which "costs" two ATP molecules.
Other kinds of cellular respiration include fermentation, where the pyruvate is converted to waste products, which when removed from the cell oxidizes the electron carriers, and anaerobic respiration wher unlike in aerobic, the oxygen is replaced by an inorganic acceptor (sulfur) is used.
- In Glycolysis (spliting sugars), Glucose, is split into two molecules of a three carbon sugar. In the process, two molecules of ATP, two molecules of pyruvic acid and two "high energy" electron carrying molecules of NADH are produced. Glycolysis can occur with or without oxygen. In the presence of oxygen (like in aerobic respiration), glycolysis is the first stage of cellular respiration. Without oxygen, glycolysis allows cells to make small amounts of ATP (fermentation).
- The Krebs Cycle (citric acid cycle) begins after the two molecules of the three carbon sugar produced in glycolysis are converted to a different compound (acetyl CoA). Through a series of intermediate steps, several compounds capable of storing electrons are produced along with two ATP molecules. These compounds, known as NAD (nicotinamide adenine dinucleotide...what is it with bio and big words?) and flavin adenine dinucleotide (FAD), are reduced in the process. These reduced forms carry the electrons to the next stage. The Krebs Cycle occurs only when oxygen is present but it doesn't use oxygen directly (in aerobic respiration).
- Electron Transport requires oxygen directly. The electron transport "chain" is a series of electron carriers in the membrane of the mitochondria in eukaryotic cells. Through a series of reactions, the earlier mentioned electrons are passed to oxygen. In the process, a gradient is formed, and eventually ATP is produced.
Maximum ATP Yields: Prokaryotic cells can yield a maximum of 38 ATP molecules while eukaryotic cells can yield a maximum of 36. In eukaryotic cells, the NADH molecules produced in glycolysis pass through the mitochondrial membrane, which "costs" two ATP molecules.
Other kinds of cellular respiration include fermentation, where the pyruvate is converted to waste products, which when removed from the cell oxidizes the electron carriers, and anaerobic respiration wher unlike in aerobic, the oxygen is replaced by an inorganic acceptor (sulfur) is used.